At UBC we are thrilled to be associated with scientists working passionately to bring innovative therapies and drugs to market. Innovations that will be the difference for how many people live their lives. As treatments are becoming more “personalized,” they encounter new hurdles which delay them from reaching many patients. These approved and available treatments can frustratingly remain just out of reach. Here’s a story about Mike and a little on why…
More Than an Annoying Itch
Meet Mike..
Mike is 47, and when he is not applying his craft of building homes and making them more comfortable, he likes to spend his days on the waterways in the Florida Keys. It’s a great life, but he can’t seem to shake a more than annoying itch and the buildup of debilitating plaques that often renders his hands incapacitated.

Mike lives with severe Atopic Dermatitis: a skin disease defined by red, itchy inflammation of the skin. He has been on Prednisone, topicals, steroid injections, and even placed in clinical trials, yet nothing has worked to allow Mike to live a normal life. Mike, a difficult case, was enrolled in a clinical trial for Xeljanz (an anti-IL-4 mAb by Pfizer), which worked to decrease his symptoms. Dupixent (similar anti-IL-4 mAb, by Regeneron/Sanofi) is FDA approved but he can’t get reimbursement for the drug because it is not on his insurance company’s formulary.
With no alternatives, the only options for Mike is to pay $2,800 a month out of pocket, or wait for 6+ months to see if this will be added to his formulary, awhile he fights through a lengthy appeal process. But even this does not guarantee access through insurance, his specific plan may not cover this specific drug. Today, Mike has to make do with the prescribed medicine that can commonly prove effective but only slightly mitigates his symptoms. It is also known to exhibit possible long-term health complications such as heart, kidney, and liver damage.
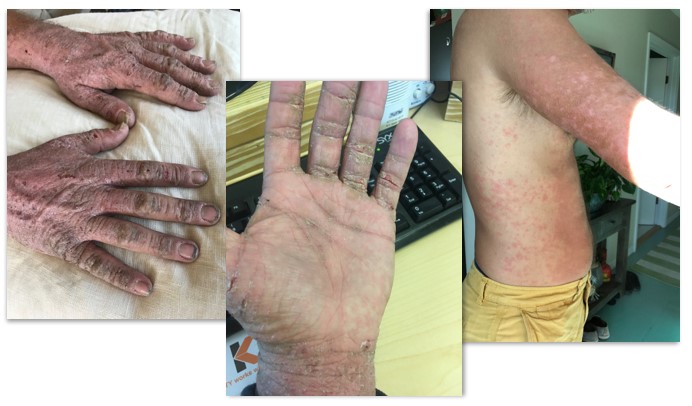
Mike’s story highlights some of the new problems associated with drug development. Specifically, the difficulty of procuring the drug that is highly beneficial or personalized to a unique patient but can also cost significantly more.
Pharmaceutical manufacturers have designed new studies to illustrate the effectiveness of their drugs and are increasing efforts communicating these results to payers. Examination of the drug’s specific needs and the therapeutic areas it serves is a primary consideration within drug development. Displaying drug and medication value through evidence will be critical in order to ensure that the use of cost management tools, do not interfere with providing care to all patients, not just those who benefit from the more generic and cost-effective treatment.
More information:
Formulary Exclusion Lists Create Challenges for Pharma and Payers Alike
Limiting the access of medications is a method for managing pharmaceutical spending, used widely by health maintenance organizations and closed health systems. As formulary exclusion lists incorporate new products and expand into new areas more patients will be challenged with access. For example, CVS’s Caremark exclusion list has grown 3.5 times and includes 134 drugs across 51 classes since 2012.
View a list of more than 80 common drugs that will be dropped from Medicare and most insurance company’s formularies in 2018.
